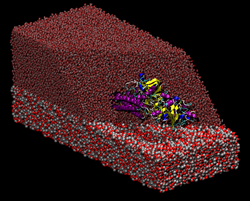
 Transport of nutrients to peripheral tissues and healing of damaged blood vessels are among the most important functions of blood. These functions involve the action of a series of proteins some of which are found in large amounts in the blood circulation. Fibrinogen is a multiprotein complex which, when activated, aggregate to form fibrin, a net-shaped molecular formation which is fundamental for the coagulation of blood following, i.e, a wound or when an extraneous body comes into contact with blood (i.e., graft implants). Thus, adsorption of fibrinogen on material surfaces play an important role in viability of those materials for implants. Transport of nutrients to peripheral tissues and healing of damaged blood vessels are among the most important functions of blood. These functions involve the action of a series of proteins some of which are found in large amounts in the blood circulation. Fibrinogen is a multiprotein complex which, when activated, aggregate to form fibrin, a net-shaped molecular formation which is fundamental for the coagulation of blood following, i.e, a wound or when an extraneous body comes into contact with blood (i.e., graft implants). Thus, adsorption of fibrinogen on material surfaces play an important role in viability of those materials for implants.
In collaboration with experimental groups in the field, we use atomistic molecular dynamics simulations to characterize the adsorption process of fibrinogen on material surfaces. Another important molecule in the blood is albumin, which mediate transport of lipids and other molecules in blood. Albumin is a multidomain protein which provides several binding sites used to bind a range of different target molecules. Target molecules (lipids, drugs, etc.) bind to albumin which act as a transporter, and are then released where needed by blood circulation. Here we use molecular dynamics simulations to study the binding modes of several lipids to Albumin and the kinetics of lipid release/uptake. If you are interested, please contact Friederike Schmid or Giovanni Settanni.
|