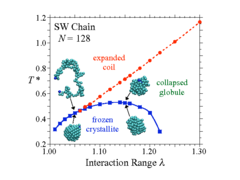
 A single polymer chain in a poor solvent may collapse into a dense fluid globule, but it may instead also crystallize: By extensive simulations with the Wang-Landau algorithm we have shown that the crystal is favorable if the range of the attractive interactions between the monomers exceeds the range of the repulsions only slightly. These findings may be useful to understand scenarios for protein crystallization. The resulting structure is also modified when an attractive substrate surface is present, and/or when one considers a semiflexible rather than a flexible polymer: then liquid-crystal-line ordering comes into play. Single chains then may collapse forming torodial or plate-like strucutures, or lamellae attached to walls. Multichain systems, or semi-flexible polymers, however, are found to undergo isotropic to nematic transitions, similar to systems of hard rods. In the presence of confinement into thin films by hard walls, "capillary normalization" (i.e. wall-induced nematic order) is found. This research is carried out in collaboration with V. A. Ivanov (Moscow State University), J. Luettmer-Strathmann (The University of Akron), M. P. Taylor (Hiram College), and W. Paul (Martin Luther Universität Halle.) For more Information, please contact Kurt Binder . A single polymer chain in a poor solvent may collapse into a dense fluid globule, but it may instead also crystallize: By extensive simulations with the Wang-Landau algorithm we have shown that the crystal is favorable if the range of the attractive interactions between the monomers exceeds the range of the repulsions only slightly. These findings may be useful to understand scenarios for protein crystallization. The resulting structure is also modified when an attractive substrate surface is present, and/or when one considers a semiflexible rather than a flexible polymer: then liquid-crystal-line ordering comes into play. Single chains then may collapse forming torodial or plate-like strucutures, or lamellae attached to walls. Multichain systems, or semi-flexible polymers, however, are found to undergo isotropic to nematic transitions, similar to systems of hard rods. In the presence of confinement into thin films by hard walls, "capillary normalization" (i.e. wall-induced nematic order) is found. This research is carried out in collaboration with V. A. Ivanov (Moscow State University), J. Luettmer-Strathmann (The University of Akron), M. P. Taylor (Hiram College), and W. Paul (Martin Luther Universität Halle.) For more Information, please contact Kurt Binder .
|